Postexposure Prophylaxis (PEP) Protects Against Rabies
Protective measures, known as postexposure prophylaxis or PEP, are the only proven way to avoid the onset of rabies. There are 3 parts to this process. All of them are necessary.1,2
Protective measures, known as postexposure prophylaxis or PEP, are the only proven way to avoid the onset of rabies. There are 3 parts to this process. All of them are necessary.1,2
It makes sense that any animal bite or wound, no matter whether there’s a risk of rabies exposure or not, should be treated as a serious injury. Your doctor may want to check for nerve or tendon damage. Wounds should be cleaned by flushing with soap and water. An iodine solution can be used to minimize the risk of infection. Care should be taken to avoid any additional damage to surrounding skin or tissue. A tetanus vaccine should also be considered as part of standard wound care.1
This important step needs to be administered quickly before the rabies virus can take hold.
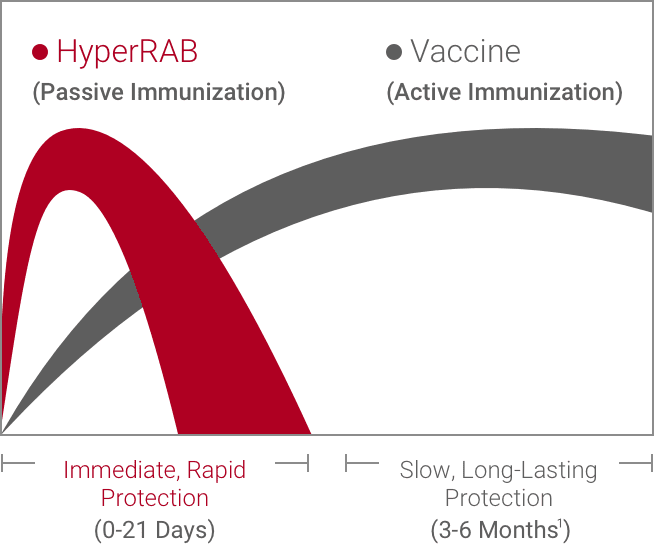
An HRIG like HyperRAB® (rabies immune globulin [human]) provides critical, immediate protection. It’s given to individuals who have not been previously vaccinated.
The most common adverse reactions in >5% of patients during clinical trials were injection-site pain, headache, injection-site nodule, abdominal pain, diarrhea, gas, nasal congestion, and oropharyngeal pain.5
The HRIG is usually administered at the same time as the first dose of the vaccine but can be given up to 7 days later. Although vaccines can protect for years, they may take weeks to become effective. An HRIG like HyperRAB allows the vaccine time to establish active immunity.1,4
HyperRAB is made from plasma from human donors who have larger than normal amounts of antibodies and is injected into the body at the wound site. If the wound is small or in a difficult part of the body to reach, your doctor will inject as much as feasible at the wound site, then administer the rest at another location, like your upper arm.1,4
In addition to the HRIG, which provides immediate protection, a vaccine is administered to build active, long-lasting immunity. The rabies vaccine is usually given as a series of 4 shots.4
If the animal that caused the wound was captured, and diagnostic testing on the animal proves it was not rabid, then the postexposure prophylaxis measures may be stopped.1
Once you have symptoms, it’s almost certainly fatal.1
If you have been exposed, get postexposure prophylaxis treatment right away.
Important Safety Information for HyperRAB® (rabies immune globulin [human])
Indication and Usage
HyperRAB® (rabies immune globulin [human]) is indicated for postexposure prophylaxis, along with rabies vaccine, for all persons suspected of exposure to rabies.
Limitations of Use
Persons who have been previously immunized with rabies vaccine and have a confirmed adequate rabies antibody titer should receive only vaccine.
For unvaccinated persons, the combination of HyperRAB and vaccine is recommended for both bite and nonbite exposures regardless of the time interval between exposure and initiation of postexposure prophylaxis.
Beyond 7 days (after the first vaccine dose), HyperRAB is not indicated since an antibody response to vaccine is presumed to have occurred.
Important Safety Information
For infiltration and intramuscular use only.
Severe hypersensitivity reactions may occur with HyperRAB. Patients with a history of prior systemic allergic reactions to human immunoglobulin preparations are at a greater risk of developing severe hypersensitivity and anaphylactic reactions. Have epinephrine available for treatment of acute allergic symptoms, should they occur.
HyperRAB is made from human blood and may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
The most common adverse reactions in >5% of subjects during clinical trials were injection-site pain, headache, injection-site nodule, abdominal pain, diarrhea, flatulence, nasal congestion, and oropharyngeal pain.
Do not administer repeated doses of HyperRAB once vaccine treatment has been initiated as this could prevent the full expression of active immunity expected from the rabies vaccine.
Other antibodies in the HyperRAB preparation may interfere with the response to live vaccines such as measles, mumps, polio, or rubella. Defer immunization with live vaccines for 4 months after HyperRAB administration.
Please see full Prescribing Information for HyperRAB.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References